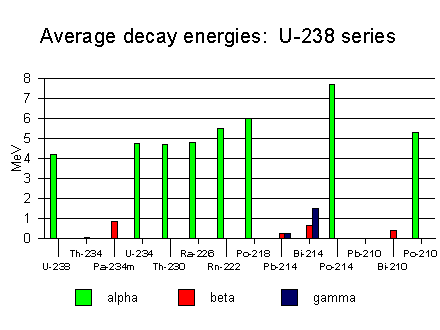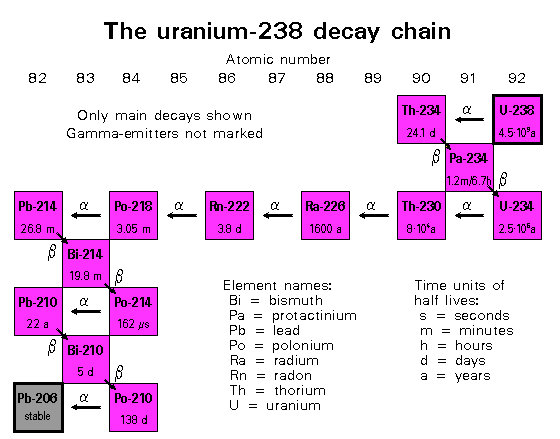

238U goes through a series of decays indicated above. The alpha decays will not be registered by the Geiger counter, because the alpha particles are easily absorbed in material. The graph below shows the average energy of the particles emitted in the decay series of 238U. For reference, the range of a 5 MeV alpha particle in air is 4.368E-03 g/cm2. This number has to be divided by the density of air 1.25 kg/m3 to get the distance that an alpha particle will travel in air before it looses all its kinetic energy. So, an alpha particle originating from a decay in the 238U series will travel only about 3.5 cm before being stopped. The electrons from the beta decay interact much less with the material, hence their range is larger than that of the alpha particles. In air, it is about 0.2 g/cm2 for a 0.5 MeV electron. These electrons will make the Geiger counter click!